1. Introduction
In gravimetric preparation of parenteral medications, hospital pharmacists rightfully expect a high level of accuracy. How much fluid has actually been added? Does the measured weight correspond to the intended amount? And how can one know whether the result can be trusted?
This whitepaper is written for pharmacists who seek insight into the accuracy and limitations of an automated system like The Compounder. It explains how gravimetry is applied, how measurements are formed, and how regulatory standards such as the e-value (MID) and d-value (display resolution) influence the interpretation of results. It also addresses why small deviations are sometimes inevitable, and how volume, bag size and total weight influence the assessment of a preparation.
2. How the system works
2.1 From signal to weight
Each scale in The Compounder contains a load cell combined with an ADC (analog-to-digital converter). The load cell converts mechanical deformation—caused by weight—into a small voltage signal (expressed in microvolts per volt). This signal is then digitized by the ADC.That digital value is converted into a mass using a previously established calibration curve. The system displays this mass in increments of 0.02 g (the d-value). This resolution is fine-grained and derived from the underlying analog signal from the load cell. The calibration line translates this signal into weight. A simplified example: at full capacity (1500 g), the load cell might output 10,000 μV. This equates to about 6.7 μV per gram. A 0.02 g step corresponds to just 0.13 μV—still a technically measurable change. This illustrates that d-values—while below the formal e-limit—are based on real, linear signals. These intermediary values (between formal e-steps of 0.2 g) are thus technically accurate.Legally, only deviations of 0.2 g or more (the e-value) may be used for formal decisions such as batch release. However, d-values of 0.02 g are based on reliable signal interpretation and provide a technically traceable indicative value.
2.2 The practical workflow
During a preparation in The Compounder, the weighing process proceeds as follows:
- A container (e.g. IV bag in holder) is placed on the scale.
- The scale is tared to 0.000 g.
- The required liquid (with known density) is automatically added.
- The system calculates the difference in weight before and after addition.
- The measured weight is compared with the expected value, and the degree of match is displayed.
The system does not evaluate the total weight of the end product, but the net amount added, based on differential weighing.
3. Classification and Certification
The Compounder is officially classified as an Automatic Weighing Instrument (AWI), specifically an automatic catchweigher, certified under the European Measuring Instruments Directive (MID 2014/32/EU, Annex MI-006). Evaluation was carried out according to international standard OIML R51 (2006), and confirmed under type certification ER12601R0 by NMi Certin. The system includes ten scales with the following specifications:
- e = 0.2 g: the verification interval. Only deviations of this magnitude or greater may be used for formal compliance or acceptance.
- d = 0.02 g: the display resolution. This is the smallest increment shown but may not be used for legal decisions.
- Weighing range: 0–1500 g: the full operational capacity per scale.
- Class Y(II): the applied accuracy class under OIML R51.
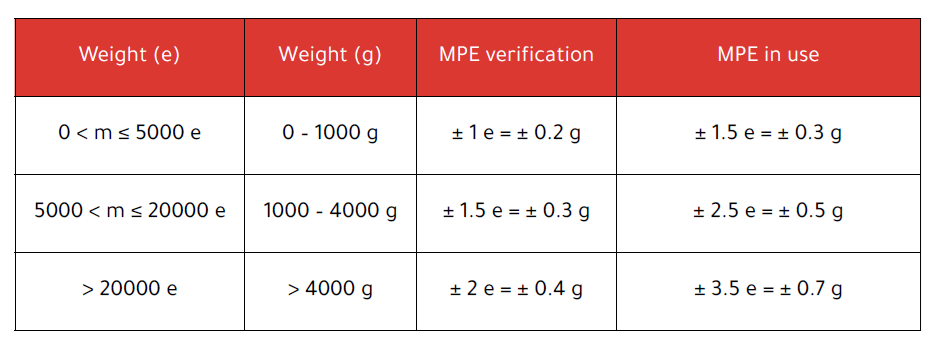
4 MPE limits under OIML R51 (Y(II))
The Maximum Permissible Error (MPE) depends on the applied weight (expressed in e-units) and is defined in Table 5 of OIML R51 for class Y(II):
For The Compounder (max 1500 g), the first two rows apply. These MPE values apply to both net difference and direct readings. The table shows the tolerances that apply in service, according to OIML R51. These are slightly more lenient than initial verification tolerances and define the legally acceptable error during real use.
5. Practical Interpretation of Measurements and Tolerances
The display of The Compounder shows values in increments of 0.02 g (the d-value). This visual resolution is based on actual measurements from the load cell. Deviations smaller than the e-value (0.2 g) may not be used for formal acceptance or rejection under MID. However, they are technically valid and may serve as internal quality indicators.
An important concept in interpretation is relative error. The MPE is absolute—it does not scale with the target volume. This means that the same absolute error has a larger impact when the volume added is small. For example, a ±0.2 g deviation on a 50 g addition is 0.4%, but on a 5 g addition it represents a 4% deviation.
Total weight on the scale also plays a role: higher load means higher allowed error. Under OIML R51 Class Y(II):
- Up to 1000 g (≤ 5000 e): MPE verification = ±0.2 g, in use = ±0.3 g
- 1000 – 1500 g: MPE verification = ±0.3 g, in use = ±0.5 g
Example: Adding 50 ml to a 100 ml IV bag (approx. 150 g): allowed deviation = ±0.2 g. Same addition to a 1000 ml bag (approx. 1050 g): allowed deviation = ±0.3 g.
Allowed error thus depends on both the added volume and the total weight—two factors that determine how to interpret a dosing result. These insights help ensure responsible use of the system in daily pharmacy practice.
The following table shows how the relative error (%) varies depending on total weight and added volume, using in-service MPE values:
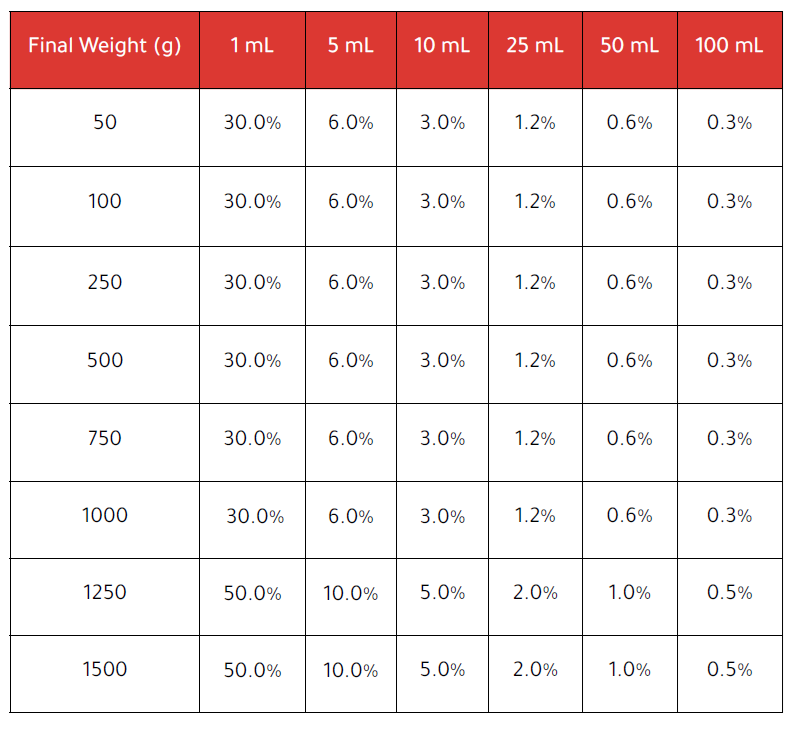
While these margins are legally acceptable, they do not reflect actual performance.Pharmacists seeking further assurance can verify system accuracy using control weights (e.g.F2 class). The Compounder includes a test routine to support this type of check.
6. Conclusion
The Compounder is certified as an automatic catchweigher and complies with all MID and OIML R51 requirements. Pharmacists can rely on a system that is accurate, reproducible, and verifiable—as long as the interpretation boundaries are respected. By understanding these boundaries, pharmacists can make optimal use of what the system can—and cannot—deliver for safe, controlled preparation.